- Drug Topics November/December 2024
- Volume 168
- Issue 08
Key Recommendations for 2024-2025 Respiratory Virus Season | NCPA 2024
The first of a 2-part presentation focused on vaccines for COVID-19, flu, RSV, and pneumococcal disease.
Respiratory virus season is here, according to David Ha, PharmD, BCIDP, but the worst is not yet upon us. The CDC has predicted that this year’s respiratory season is going to look similar—"or maybe slightly less intense”—compared to last year’s season, “but we’ve been fooled before,” Ha said.
In the first part of his a 2-part presentation at this year’s National Community Pharmacists Association (NCPA) Annual Meeting and Exposition, Ha—Infectious Diseases Manager at Stanford Health Care—provided a comprehensive overview of vaccines for 4 common respiratory viruses and touched on the latest updates from the October 2024 CDC Advisory Committee on Immunization Practices (ACIP) meeting, held just days before the conference.
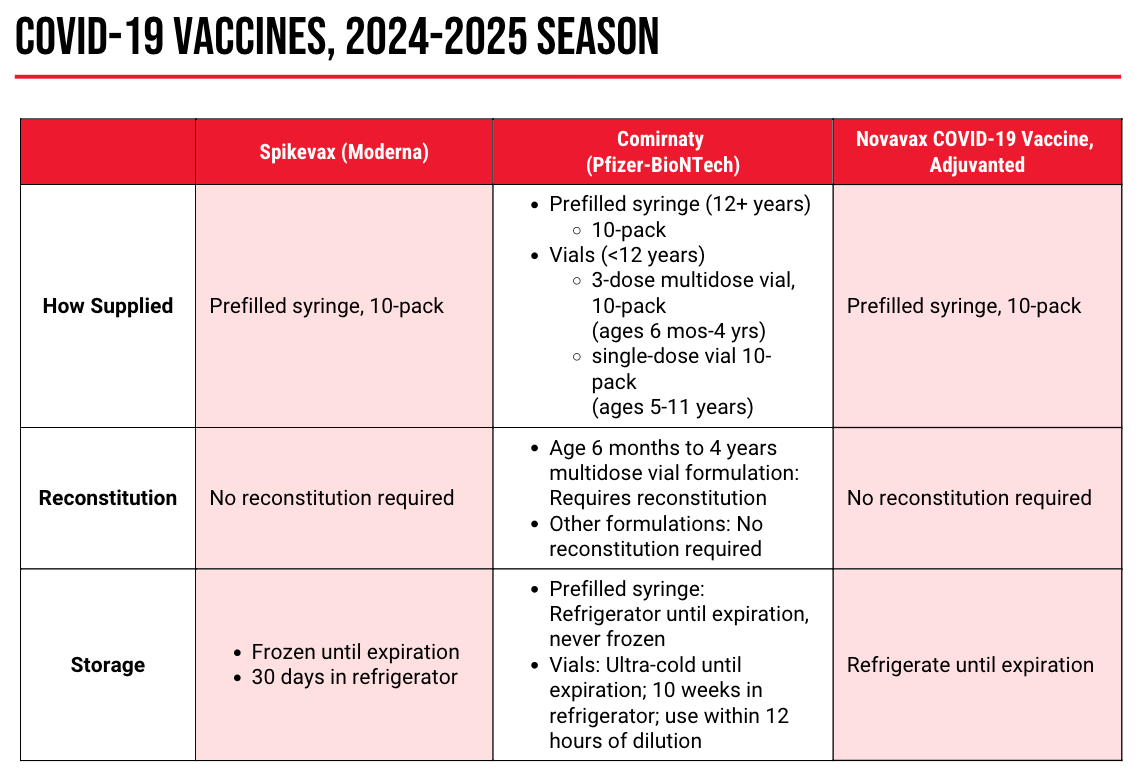
COVID-19
The 2024-2025 COVID-19 vaccine is not a new vaccine, but a strain change. This year’s shots are monovalent, targeting the Omicron JN.1 lineage of SARS-CoV-2. The FDA authorized the new versions of these vaccines—mRNA versions from Moderna and Pfizer, and a protein-based version from Novavax—in August 2024.
Despite the availability of new shots, the low rates of vaccination may be indicative of another phenomenon: COVID-19 fatigue. “You may be getting questions…from your patients. Year after year, new variants, ‘Does it still make sense for me to continue to get vaccinated?’” Ha said. One way to address these questions? Safety and efficacy data from the previous season.
Across all age groups, the 2023-2024 COVID-19 vaccine—a monovalent vaccine targeting the SARS-CoV-2 XBB.1.5 sublineage—was effective against COVID-19 infection, emergency department and urgent care visits, and hospitalizations. What’s more, this efficacy persisted for at least 6 months after immunization, with waning observed around the 4- to-6-month mark. Safety data showed similar rates of adverse reactions to the 2023-2024 vaccine as in prior seasons, and no signal for myocarditis or pericarditis in men aged 12 to 39 years.
“Vaccination rates have declined, as I’m sure you’re aware, in the past [few] years, especially since the peak of the COVID-19 pandemic,” Ha said. Data from earlier this year indicate that more than one-third or less of eligible individuals reported that they are up to date with their COVID-19 vaccination.
But with more than 8 in 10 Americans
The CDC Advisory Committee on Immunization Practices (ACIP) met on October 23 and 24, 2024 to make recommendations for the 2024-2025 COVID-19 vaccine. At the time of Ha’s presentation (and the writing of this article), those changes had not yet been made to the CDC’s website. Major changes from the ACIP 2024 October update include:
- Recommendation of a second dose of the COVID-19 vaccine for adults aged 65 years and older. Ideally, this dose should be administered 6 months from the initial or prior dose.
- In patients 6 months and older who are moderately to severely immunocompromised, 2 doses are recommended. A third—or more—dose may be recommended based on shared clinical decision making.
- Although it is ideal for patients to receive their vaccine from same manufacturer, mRNA vaccines are interchangeable if the same dose is not available.
- New this year, for those who receive the Novavax vaccine: The initial 2-dose series should be with the Novavax vaccine, but if it has been more than 8 weeks since the first dose, the mRNA vaccine can be substituted, if that is the only immunization available
- Self-attestation of immunocompromise status is sufficient, and specific documentation is not required. Conditions include organ transplant, cancer, chronic kidney or hematologic disease, HIV, and/or primary immunodeficiency, among others.
There is no specific timing recommended for COVID-19 vaccination. Protection is at its highest within the first weeks to months after vaccination; therefore, high-risk patients should not wait. Co-administration of the COVID-19 vaccine with other respiratory virus vaccines is encouraged.
Influenza
“We haven’t seen influenza season kicking into high gear yet, but that’s certainly going to be coming,” Ha said. “It’s late October; the patients [who] haven’t had their flu shot yet, they should get it ASAP.”
READ MORE:
The 2024-2025 flu vaccine has transitioned from a quadrivalent to a trivalent vaccine. “This is an amazing story,” Ha said. “Due to a lot of the COVID-19 interventions, we have eliminated influenza B/Yamagata from circulation; it just hasn’t existed for at least the past season.” This year’s formulation targets the A/H1N1 (Victoria) strain, the A/H3N2 (Thailand) strain, and the B/Austria (Victoria-like lineage) strain.
Ha noted that the influenza vaccine recommendations—largely unchanged by ACIP—are “fairly straightforward”: the CDC still recommends routine vaccination for all individuals aged 6 months or older and individuals aged 65 years or older should preferentially receive a high-dose, adjuvanted, or recombinant vaccine. New for 2024-2025 is the recommendation that individuals who received solid-organ transplants may receive high-dose or adjuvanted vaccines.
“Egg allergy is not a contraindication,” Ha said. “It doesn’t matter what formulation, it doesn’t matter what reaction. The CDC says that you don’t even need additional safety measures; the risk is vanishingly low. It is nearly undetectable because of the infinitesimally small amount of egg protein that is incorporated into these vaccines.”
Self-Administered Influenza Vaccines
New in flu vaccination options is the first and only FDA-approved self- or caregiver-administered flu vaccine,
“This is especially exciting for our pediatric patients,” Ha said, a population that can be difficult to vaccinate.
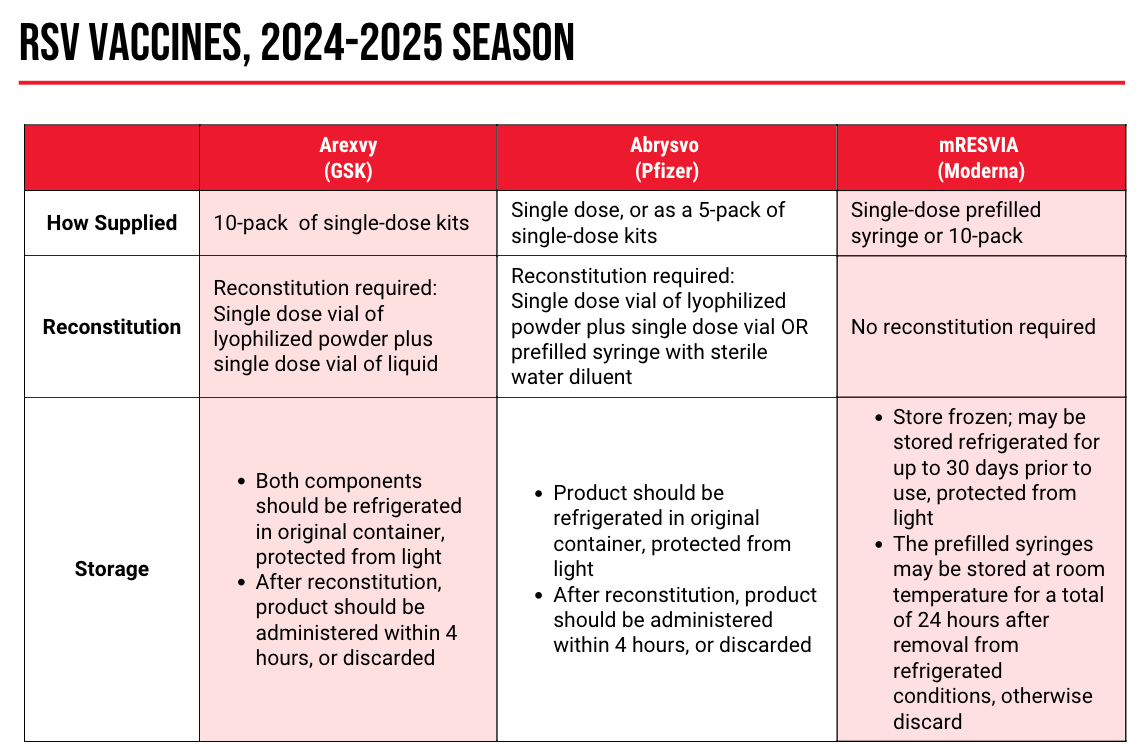
Respiratory Syncytial Virus (RSV)
“I get this a lot from patients: Why is RSV a big deal?” said Ha. “I think this comes from the fact that we’ve talked a lot about COVID, people have heard a lot about influenza, [but] they haven’t heard a lot about RSV. It hasn’t made its way into the news as much as other viruses.”
READ MORE:
Despite the lack of public knowledge, RSV vaccination is crucial: on a yearly basis, the vaccine is responsible for between 6000 and 10,000 deaths, 60,000 and 160,000 hospitalizations, and 0.9 to 1.4 million medical encounters, with older adults—especially those living in long-term care facilities or those with cardiopulmonary conditions—are at risk for severe RSV.
Real-world efficacy data, presented at the June 2024 ACIP meeting, “mirrors what we saw in the clinical trials,” Ha said. “This is a fantastically effective vaccine that has quite a bit of durability.”
At the June 2024 meeting, ACIP made the following recommendations for RSV vaccination in older individuals:
- Adults aged 75 years and older who are unvaccinated should receive routine RSV vaccination.
- Adults aged 60 to 74 years who are unvaccinated should receive routine vaccination if they are at high risk for severe disease—for example, those with chronic medical conditions or who live in a nursing home residence or long-term care facility.
- For individuals who were previously vaccinated, no repeat vaccine is indicated.
One common question Ha sees is around the recommendation for adults aged 60 to 74 years who are otherwise healthy. “Based on last season’s recommendations—based on shared clinical decision making—there was an opportunity for those, even without comorbidities, to get vaccinated. That is no longer a CDC recommendation.”
“But what I will say is, this is evolving over time,” Ha added. “CDC is going to be evaluating a broader recommendation for vaccination. I personally would love to see more of a routine recommendation for those that are 60 [years] and older.”
Only 1 RSV vaccine is indicated for use in pregnant women, Pfizer’s
A common question within this patient population is around the
The CDC recommends that pregnant women who are 32 to 36 weeks’ gestational age should receive 1 dose of Abrysvo if not previously received. Administration is seasonal (between September and January in most of the US) and only 1 lifetime dose should be given.
Pneumococcal Conjugate Vaccines
Finally, Ha touched on pneumococcal vaccination, for which there was a notable update at the October 2024 ACIP meeting.
Merck’s PCV21 (21-valent pneumococcal conjugate vaccine, Capvaxive ) is the newest pneumococcal vaccine available, approved by the FDA in June 2024. “It’s not just an extended spectrum of serogroups, it really is a different type of vaccines,” Ha said. “It really was designed from the ground up to target the actively circulating strains… PCV21 provides a broad range of coverage compared to the other conjugate vaccines.”
At the ACIP meeting, committee members voted to
Additional recommendations include routine vaccination for:
- Individuals aged 19 to 64 years with chronic medical conditions who have never received a pneumococcal conjugate vaccine
- Individuals aged 19 years and older who received PCV13 (13-valent pneumococcal conjugate vaccine, Prevnar 13) or PCV7 (7-valent pneumococcal vaccine, Prevnar), but not PPSV23 (pneumococcal polysaccharide vaccine; incomplete series)
- Children aged 2 to 18 years with chronic medical conditions
- Infants and children
- Immunocompromised individuals
- Individuals with a cerebrospinal fluid leak or cochlear implant.
- Shared clinical decision making can be implemented for adults aged 65 or older who received the PCV13 vaccine at any age plus the PPSV23 vaccine at age 65 or older.
At the time of Ha’s presentation (and the writing of this article), those changes had not yet been made to the CDC’s website.
Stay tuned for our coverage of Part 2 of this presentation. Until then, check out the rest of our
Coverage of the National Community Pharmacists Association 2024 Annual Convention and Expo was supported by Red Sail, with independent editorial content creation on-site by the Drug Topics team.
References
1. Ha, D. Inhale, exhale, immunize: Navigating the latest in respiratory vaccines. Presented at: National Community Pharmacists Association 2024 Annual Convention and Expo; October 26-29, 2024; Columbus, OH.
Articles in this issue
about 1 year ago
OTC Product Roundup: Sleep Aidsabout 1 year ago
It’s Time to Staunch Our Self-Inflicted Woundsabout 1 year ago
A Look Back at the Affordable Care Actabout 1 year ago
Truth or Trend? Equipping Patients to Combat Misinformationabout 1 year ago
Q&A: Pharmacists Champion Nonopioid Pain Management StrategiesNewsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.