
However, there is no association between cortisol and tau pathology.

However, there is no association between cortisol and tau pathology.

Researchers conduct a scoping review to update and determine what the best available options are for treating patients with skin-picking disorder.

Pharmacies face mounting challenges amid increased competition, rising operational costs, and complex reimbursement models.

In children aged 5 to 17 with skin disorders, researchers explore the risk of developing psychosocial comorbidities, sleep disturbances, and common related symptoms.

Glucagon-like peptide-1 receptor agonists have been associated with improvements in quality of life (QOL), restrained eating, and emotional eating behavior.
Jeff Goad, PharmD, MPH, discusses hesitancy for hepatitis vaccines and the necessary advice pharmacists can utilize to help patients manage these misconceptions.
Novavax’s COVID-19 vaccine has been available in the US under emergency use authorization since July 2022.

Researchers conducted a clinical review detailing the intersection of dermatology and suicide, explaining why skin conditions lead to increased risk of poor mental health.

Investigators note that the long-term effects of attention deficit/hyperactivity disorder (ADHD) medication for children remain lacking.

The announcement comes shortly after the company announced it had filed its second chapter 11 bankruptcy in under 2 years.

Drug Market experts joined Drug Topics to discuss the rise in direct-to-consumer drug advertising and what it means for patients around the world.
Jeff Goad, PharmD, MPH, discusses hepatitis viruses and the current vaccination schedules available for patients to stay protected.

Dihydroergotamine (Brekiya) is an acute treatment to help relieve migraine attacks, and it can come in nasal sprays, injections, and intravenous infusions.
The investigators hope to pursue a human vaccine in order to improve upon the already existing vaccines.

Ronna Hauser, PharmD, Senior Vice President of Policy & Pharmacy Affairs at NCPA, provides insights into President Trump’s most recent executive order regarding manufacturer drug prices.

Investigators report that patients experience weight loss with a reduced weekly dosage of the glucagon-like peptide-1 (GLP-1).

NBC News Medical Analyst Vin Gupta, MD, joins Drug Topics at NCPDP 2025 to discuss the technological evolution of the pharmacy profession.

Attorneys from The Phoenix Law Group joined Drug Topics at NCPDP 2025 to discuss the current state of pharmacy benefit manager reform and where it may end up in the near future.
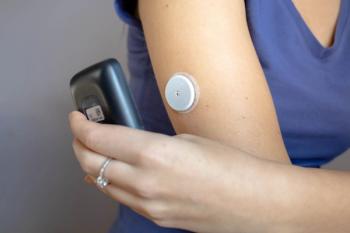
Abbott’s continuous glucose monitoring system is the first to show a reduction in cardiovascular complication severity.

The NCPDP’s Executive Vice President of Innovation & Standards joins Drug Topics to discuss how AI has been making itself known within the pharmacy profession.

However, investigators find many gaps remaining in care for patients in relation to race, ethnicity, and disabilities.

Investigators also find that patients diagnosed with type 1 diabetes later in life do not have a better prognosis compared to those diagnosed earlier.

Rick Sage, Executive Vice President of Innovation & Standards at NCPDP, discusses his role and how his team is using innovation to support the pharmacy profession.

Dae Lee, PharmD, Esq, CPBS and Lucas Morgan, Esq, discuss PBM and board of pharmacy enforcement actions, trends in PBM audits, and more.

Larry King, PharmD, joins Drug Topics at NCPDP 2025 to discuss pharmacy interoperability, changes in its regulations, and how technology will fall into place within the data infrastructure.

In part 3 of our interview with Vin Gupta, MD, he discusses pharmacists’ role in remote patient care amid a growing technological infrastructure.
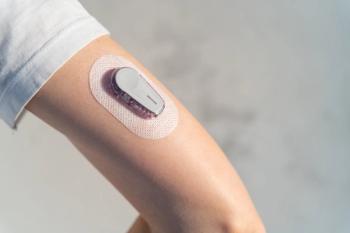
Investigators also found that nocturnal hypoglycemia is a strong predictor of daytime hypoglycemia.

Pharmacist-led interventions improve medication adherence compared with the usual care for patients with heart failure and type 2 diabetes.

In the second part of our interview with Miranda Rochol, she discusses challenges regarding digital prescription solutions and the financial burdens these solutions are designed for pharmacists to overcome.
In her expertise as a leader with the American Pharmacists Association, Brigid Groves, PharmD, MS, provides insights into her work with geographical information systems and maps.