The Advisory Committee on Immunization Practices voted to lower the pneumococcal recommendation to 50 years in October 2024.

The Advisory Committee on Immunization Practices voted to lower the pneumococcal recommendation to 50 years in October 2024.

In this episode, Natalie DiPietro Mager, PharmD, PhD speaks with David Bright, PharmD, MBA, professor at Ferris State University College of Pharmacy.

Hepatitis B and liver functions have been found to be independent risk factors for gestational diabetes and are found to have the highest prevalence of abnormalities.

Widaplik is a single pill combination of telmisartan, amlodipine and indapamide used to lower blood pressure in adult patients.

There were more fires in the span of a 13-year period from 2005 to 2018 than in the previous 2 decades.
Chronic pain is associated with a significant financial burden, with an estimated cost placed at $650 billion annually in the US.
Building trust with patients through fact-based information could help boost vaccination rates to the desirable coverage.
Social media platforms provide an opportunity for pharmacists to create accurate content on inflammatory bowel disease for younger patients.

Investigators found that longer durations of GLP-1 use were associated with increased risk of nAMD.
Pharmacy team members can help patients prepare for upcoming travels, ensuring they are up to date with the recommended vaccines for their locations.
Lauren Angelo, PharmD, MBA, associate dean of academic affairs at Rosalind Franklin University of Medicine and Science, highlights significant concerns about the evolving landscape of COVID-19 vaccine policy.

In this episode of the podcast, Shawn and Jesse discuss the benefits of being easy to do business with.
Investigators found that simvastatin added to escitalopram for patients with obesity and major depressive disorder did not have increased antidepressive effects.

Postmenopausal women who ate mango daily for 2 weeks had lower blood pressure measures and lower total cholesterol.
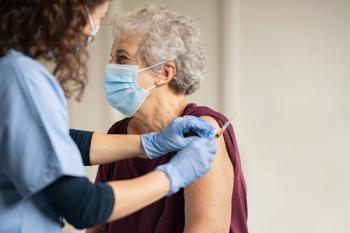
Lauren Angelo, PharmD, MBA, associate dean of academic affairs at Rosalind Franklin University of Medicine and Science, discusses the changing recommendations for COVID-19 vaccination.

A significant number of patients remain unaware of their diabetes, signaling a need for better diagnostic efforts.
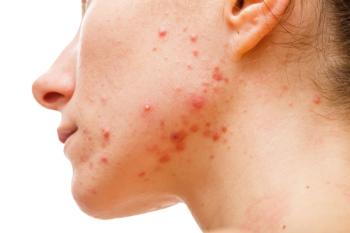
ASC40 blocks facial sebum production by inhibiting de novo lipogenesis in sebocytes and inflammation by decreasing cytokine secretion and Th17 differentiation.

The associations and strengths of these links varied by the complication type, including diabetic nephropathy, neuropathy, and retinopathy.

The primary challenges include disruption in medication supply and increased demand for essential medications due to heightened health concerns.

A conversation with Erin Albert, MBA, PharmD, JD, DASPL, chief of pharmacy relations, network and privacy, at Cost Plus Drugs.

Social determinants of health have significant impacts on health and quality of life, but little is known about the impact during menopause.
Esaxerenone is a nonsteroidal mineralocorticoid receptor blocker that is being studied for its effectiveness for diabetes-associated mineralocorticoid receptor-related hypertension.

In the aftermath of natural disasters, pharmacists play an essential role in the disaster relief team.
The most common barriers to vaccination included finances, logistics, availability of information, and hesitancy or refusal.
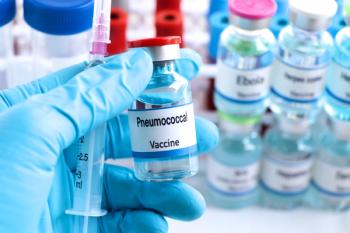
Invasive pneumococcal disease is a cause of morbidity and mortality in the US, despite the availability of vaccination against the disease.

Results from a phase 2 trial showed that semaglutide combined with trevogrumab, with or without garetosmab, helped patients preserve lean mass and achieve greater fat loss.

Pharmacists are indispensable members of the disaster relief team, playing a crucial role in ensuring the continuity of care during and after a natural disaster.

The new approval is in line with recommendations that suggest booster vaccination for older adults and patients aged 12 to 64 with at least 1 or more underlying risk factors.

The event will focus on revenue stream diversification, providing independent pharmacies with seeds of innovation and practical tools to cultivate new avenues for growth in pharmacy.

Joey Mattingly, PharmD, MBA, PhD, and Marta Wosinska, PhD, discussed what pharmacy businesses can do to prepare for the potential implementation of tariffs on pharmaceutical products.