
Researchers explored the global disease burden of allergic-related skin diseases from 1990 to 2021 and projected future disease trends.

Researchers explored the global disease burden of allergic-related skin diseases from 1990 to 2021 and projected future disease trends.

Buddy Carter, PBM, US Representative (R-GA), emphasizes the critical role of pharmacists, particularly during the COVID-19 pandemic, and advocates for provider status recognition.
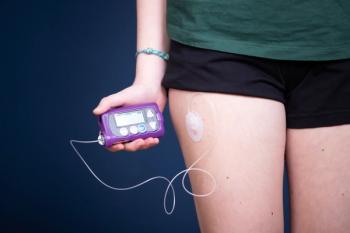
Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, joins Drug Topics to discuss her extensive background in managing patients’ insulin regimens.

Pharmacists can transform from only being prescription dispensers to health care hubs that offer comprehensive patient care and generate innovative revenue streams.

With federal reform continuing to stall, Drug Topics caught up with Iowa State Representative Brett Barker, PharmD, to discuss how his state is regulating pharmacy benefit managers.

Researchers conducted a content analysis to identify pharmacy benefit manager legislation supported by the NCPA and assessed whether or not it would benefit rural pharmacies and patients.

The FDA set a Prescription Drug User Fee Act (PDUFA) date of April 28, 2026.

Researchers explored a variety of medicinal plants used for skin-related conditions and assessed their efficacy in treating UV-induced skin damage.

The key theme, regardless of strategy, is being an engaging member of the community and stepping outside of the counter.

Women who received mindfulness-based interventions experienced improved sleep quality and reduced anxiety, depressive symptoms, and stress.

Researchers conducted a survey assessing pharmacy students’ perceptions of pharmacist burnout and how that may impact their future career paths.

Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, discusses the importance of insulin delivery and the common barriers pharmacists face in managing patients’ regimens.

In part 3 of our interview with Iowa State Representative Brett Barker, PharmD, he discusses how legislation at the state level is gradually informing decisions at the federal level.
Researchers investigated the current treatment strategies for atopic dermatitis and how they related to ideas of corticophobia among providers.

CPESN USA announces the recipients of its 2025 CPESN Most Engaged Pharmacy Awards and recognizes 32 pharmacies from across America.

In this episode, Natalie DiPietro Mager, PharmD, PhD, MPH, speaks with Lindsey Ferraro, PharmD, associate professor of pharmacy practice at Ohio Northern University.
Patients with a single cardiometabolic disease have an approximately 48% greater risk of developing all-cause dementia than those without a cardiometabolic disease.

Researchers explored the effectiveness of telephonic insulin titration by a clinical pharmacist compared with in-office titration among patients in a medical residency clinic.
Brett Barker, PharmD, state representative in Iowa’s 51st District, joined Drug Topics to discuss significant implications and common misconceptions regarding Iowa’s new PBM bill.
Efsitora demonstrates noninferior reductions in hemoglobin A1c and similar rates in patients with an A1c level less than 7% compared with common daily insulin.

The therapy is now the first and only oral on-demand treatment option for the condition.

Researchers determined the impact COVID-19 interventions had on Danish children’s incidence of RSV, influenza, and invasive pneumococcal disease.

This year’s conference is taking place from July 10 to 13 in Nashville, Tennessee.
Brett Barker, PharmD, State Representative in Iowa’s 51st District, discussed Senate File 383 and how it’s intended to impact Iowa pharmacies.
Investigators also report that sodium-glucose cotransporter 2 inhibitors are not associated with thrombotic risk.

Health care professionals emphasize patient education and reliable information in the evolving vaccine recommendation landscape.

Kevin W. Cleveland, PharmD, urges evidence-based approaches to immunizations in an era of uncertainty.

Following his presentation at the American Diabetes Association’s 85th Scientific Sessions, Jon Easter caught up with Drug Topics to discuss the pharmacists’ role in diabetes management.

As state-level PBM reform heats up amid dire situations for many retail pharmacies, Walgreens Chief Pharmacy Officer Rick Gates joins Drug Topics to discuss ongoing industry challenges.
Patients who used the Bigfoot Unity Diabetes Management System had glycemic control and reduced diabetes distress.