
Nonstimulant drugs and even nonmedication options are available for use.

Nonstimulant drugs and even nonmedication options are available for use.
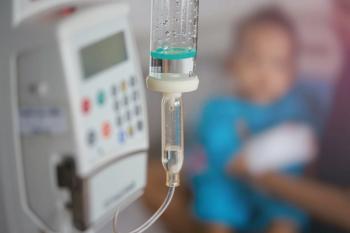
An expert offers considerations and developmentally appropriate interventions to promote high-quality, patient-centered pediatric infusion care.

The effect of acne is more than skin deep.
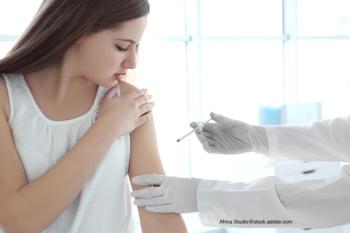
The FDA approved the pneumococcal 15-valent conjugate vaccine for the prevention of invasive pneumococcal disease in infants and children.
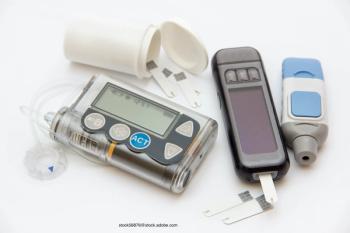
An analysis of the SEARCH for Youth in Diabetes describes historic and contemporary disparities in insulin pump use for pediatric type 1 diabetes based on racial/ethnic background, household income, and insurance type.

The FDA announced the EUA of both Moderna and Pfizer-BioNTech COVID-19 vaccines for use in children aged 6 months and older.
Prenatal use of the psychoactive drug benzodiazepines is not a major risk factor for altered neurodevelopment in early childhood, despite crossing the human blood-placenta barrier.

Sanofi and Regeneron Pharmaceuticals, Inc announced the FDA approval of dupilumab. It is now the first biologic medicine for children from 6 months to 5 years old with moderate to severe atopic dermatitis.

A survey saw that of teenagers who sought care in the ED and were prescribed outpatient treatment, fewer than half who received an STI diagnosis filled their prescriptions.

GSK plc announced FDA approval of the PRIORIX vaccine for prevention of measles, mumps, and rubella.

Pediatric patients taking 4 or more medications were more likely to experience medication errors.

Current data about naloxone use in pediatric populations is extrapolated from adult studies.

High-dose haloperidol and aprepitant were both associated with emergency department discharge.

The FreeStyle Libre 3 system, billed as the world's smallest, thinnest, and most accurate 14-day glucose sensor, received FDA clearance on May 31 and is expected to be available in participating pharmacies later this year.

While antibiotic dosing was correct, therapy duration was frequently inappropriate.
Pfizer-BioNTech reports strong immune response, high efficacy, and favorable safety in third dose of the COVID-19 vaccine.

Protection from the harsh rays of the sun is crucial for everyone—including children. Now that summer has arrived, a refresher course can go a long way in preventing skin damage and disorders due to the sun.

The proposed aid bill would give the FDA funds to increase staff and prepare for future infant formula shortages.

This week, the agency announced it is encouraging importation of infant formula from usually unutilized foreign means in hopes of increasing access to the product in the wake of the country-wide shortage.
A comparison of COVID-19 PCR tests and rapid self-tests found self-tests to be highly accurate and user-friendly for children.

Parents are on edge as the formula shortage worsens across the United States. Pediatricians, the AAP, and White House advise on available options.
Families often turn to supplementation to ensure that children are getting the necessary nutrients

The exponential rise in death rate is historic, even as adolescent drug use is low.

At the 43rd National Conference on Pediatric Health Care for nurse practitioners in Dallas, Texas, a look at the newest medications for children with attention-deficit/hyperactivity disorder.
A baseline of dosing errors can lead to a framework to improve antibiotic prescribing habits.