
An over-the-counter (OTC) progestin only pill (POP) could potentially reduce the overall number of unintended pregnancies in the United States.

An over-the-counter (OTC) progestin only pill (POP) could potentially reduce the overall number of unintended pregnancies in the United States.

The compound may also provide a synergistic effect in combination with fluconazole.

Results suggest the potential of this oral therapy to address the unmet clinical need for long-term medical treatment for endometriosis.
Prenatal use of the psychoactive drug benzodiazepines is not a major risk factor for altered neurodevelopment in early childhood, despite crossing the human blood-placenta barrier.
In June 2021, ibrexafungerp (Brexafemme; Scynexis) became the first drug approved by the U.S. Food and Drug Administration (FDA) in a novel antifungal class in more than 20 years and is now available to treat vulvovaginal candidiasis (VVC).
PCOS prevalence in girls with type 2 diabetes was almost 20%.
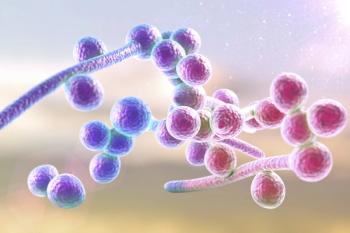
Ibrexafungerp is a promising novel, well-tolerated and effective oral 1-day treatment for acute vulvovaginal candidiasis.
While the use of combined oral contraceptives in patients with rheumatoid and autoimmune disorders have focused on safety, a recent narrative review highlighted the efficacy and benefits of these contraceptives in women with RA.

Combined oral contraceptives were found to be moderately more efficacious than placebo for treating overall premenstrual symptomatology in women with premenstrual syndrome (PMS) or premenstrual dysphoric disorder (PMDD).

Researchers evaluated the effectiveness of oral probiotics in secondary prevention of vulvovaginal infections in pregnant women.
Half of prisons and over 80% of jails in the United States allowed postpartum permanent contraception, according to a study in the journal Contraception.

The findings show that increased access to emergency contraception does not compromise use of other more effective methods or lead to an increase in reported levels of unprotected sexual intercourse, according to Hawkins.
Human papillomavirus (HPV) vaccination has significantly decreased the incidence and mortality of cervical cancer in girls and women between the ages of 15 and 24, according to a research letter in JAMA Pediatrics.
The benefits of probiotics do not necessarily transfer to the clinical setting for vulvovaginal candidiasis, according to a recent review.
One year after the close of the Increasing Access to Contraception (IAC) Learning Community, participants had sustained their efforts in support of at least 1 goal: to improve adolescent girls' and women’s access to the full range of contraceptive options.

A research letter reported that robust secretion of SARS-CoV-2 specific IgA and IgG antibodies were found in breast milk of breastfeeding women for 6 weeks after the first of 2 vaccinations against COVID-19.
Published: February 9th 2022 | Updated:
Published: November 12th 2021 | Updated: