
At this year’s American Pharmacists Association Annual Meeting & Exposition, Mark Garofoli urged pharmacists to develop strong documentation practices and go beyond baseline federal regulations to care for patients with red flags.

At this year’s American Pharmacists Association Annual Meeting & Exposition, Mark Garofoli urged pharmacists to develop strong documentation practices and go beyond baseline federal regulations to care for patients with red flags.

Pharmacists must uphold their “corresponding responsibility” to address controlled substance concerns among patients while balancing safety and respect, said Mark Garofoli at this year’s American Pharmacists Association Annual Meeting & Exposition.

The average US household spent $645 on OTC products in 2022.

Adults aged 65 years and older are the largest group of consumers of OTC medications.
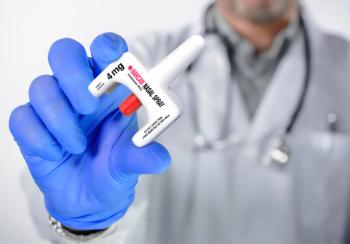
The first nonprescription, OTC naloxone nasal spray (Narcan) was approved by the FDA in March 2023.

The approval represents the first non-statin treatment indicated to lower low-density lipoprotein for primary prevention patients.

Four posters presented at this year’s American Pharmacists Association Annual Meeting & Exposition assessed the potential impact of pharmacist interventions on improving mental health outcomes.

According to posters presented at the 2024 American Pharmacists Association Annual Meeting and Exposition, pharmacists wish to play a bigger role in educating young people about the risks of vaping.

Four posters presented at the American Pharmacists Association Annual Meeting and Exposition explored how individuals and health care providers have been handling the COVID-19 pandemic.

Researchers aimed to address the adverse effects of veterans with COVID-19 diagnoses 18 months after infections were documented.

In its ongoing campaign against “scope creep,” the American Medical Association argued that pharmacists don’t receive enough training. Physicians and pharmacists alike disagree.

Farmers and welders have adapted to work with the current times. Pharmacists should consider adapting their profession as well.

Rilpivirine (Edurant Ped) effectively suppresses the HIV-1 virus in pediatric patients with HIV-1 RNA less than or equal to 100,000 copies/mL in combination with other antiretroviral therapies.
Stimulant medications are the first-line pharmacologic therapy for ADHD, but many of these medications are currently in short supply.

Researchers collected data to measure inequities in pharmacy access across the country.

Supreme Court decisions and state-level legislation have made abortion access in some states more complicated than ever before.

These OTC products are recommended to address a variety of symptoms associated with women's health issues.

The latest guidelines advise managing obesity first, then treating a patient’s other conditions to produce optimal outcomes.

APhA 2024 will take place March 22 to March 25 in Orlando, Florida.
Moderna, Merck, and Pfizer-BioNTech are leading the charge with new vaccines for respiratory viruses in development.

Despite the significant growth of biosimilars, their adoption across various therapeutic areas has remained slow.
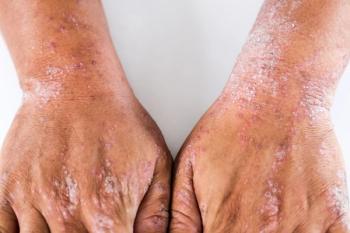
A lack of diversity in clinical trials has hampered health care providers from understanding the nuances of diseases across specific patient populations.

AstraZeneca is the second pharmaceutical company to cap out-of-pocket costs for eligible patients.
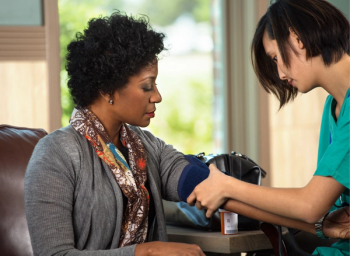
Hypertension remains a significant problem in areas of the rural South.
See what's trending in pharmacy with a preview the Drug Topics March issue.

Check out a recap of important pharmacy news you might’ve missed this week, dispensed in small doses.
Telehealth mitigates barriers like stigma, fear, and legal concerns associated with receiving opioid use disorder (OUD) care by offering a convenient and discreet treatment option.

The approval was based on data from the phase 1/2 TRANSCEND CLL 004 study, in which the CAR T cell therapy demonstrated statistically significant complete response rates.

The approval, awarded to Madrigal Pharmaceuticals, was based on phase 3 data demonstrating that resmetirom achieved broad treatment effects in patients with MASH with liver fibrosis.