
Programs increasingly cater to the uninsured and underinsured

Staying abreast of the pharmacy profession can be easier, with the right tools.
A three-year study finds that aspirin may have a role in preventing COPD flare ups.

Try your hand at new services, activism, and product offerings!
First non-insulin drug for pediatric use approved in nearly 20 years.

Type 2 diabetes mellitus is a risk factor for incident heart failure, according to a new scientific statement from the American Heart Association (AHA), and it increases the risk of morbidity and mortality in patients with established disease.
Study finds higher Ischemic events and mortality in transcatheter valve replacement patients taking non-vitamin K antagonist oral anticoagulants versus vitamin K antagonists.

Low-risk atrial fibrillation patients who take oral anticoagulants have a lower risk of dementia than those who do not, a study says.
Changes to vaccine recommendations for older adults may mean they have to pay for pneumococcal vaccine themselves.

Virus-like particle research could lead to a vaccine against opioid addiction.
Indications include breast cancer, metastatic gastric cancer, and metastatic gastroesophageal junction adenocarcinoma.

Both responder and remitter patients experienced a statistically significant longer time to relapse with esketamine compared to the placebo

Billing solutions and entrepreneurs needed.

DIR abuse might require Presidential and Congressional intervention.

Be an expert at evaluating clinical studies with some simple tools.
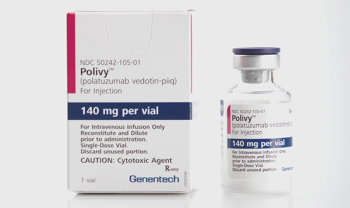
Drug targets the CD79b protein on B-cells.

Comply with state and federal regulations on dispensing and handling controls to avoid possible disciplinary or DEA actions and penalties.
Organization calls for state-level legislative and regulatory revisions.

Figuring out the equations for quality technicians.
Results from a new Massachusetts Health Policy Commission report.
Two studies outline the risks of shingles for patients with IBD.

A study finds herpes viruses can reemerge under the stresses of spaceflight.

Pharmacists can earn up to 27 hours of CE.

CVS will expand its health offerings, including nutrition and weight loss.

Results of a recent survey suggest employee-based drug abuse is a concern for many healthcare providers, though they don’t believe it can happen in their facilities.
Zerbaxa is a cephalosporin antibacterial and beta-lactamase inhibitor combination drug originally approved for abdominal and urinary tract infections.

Patients on Emgality experienced an average of 8.7 fewer weekly episodic cluster headaches.

Training programs reflect the changing practice of pharmacies.

Improve your counseling skills to make the most of medication therapy management in your community pharmacy.

The agreement will bring real-world data to cancer treatment development.