
SingleCare, in partnership with GeniusRx, launched the delivery service with the goal of improving access to and affordability of popular medications.

SingleCare, in partnership with GeniusRx, launched the delivery service with the goal of improving access to and affordability of popular medications.

An overview of the use of satralizumab-mwge (Enspryng; Genentech) in neuromyelitis optica spectrum disorder (NMOSD).

Loteprednol etabonate ophthalmic suspension (Eysuvis; Kala Pharmaceuticals) 0.25% is the first ocular corticosteroid indicated for dry eye disease.

Remdesivir (Veklury; Gilead) is the first and only fully FDA-approved treatment for COVID-19.
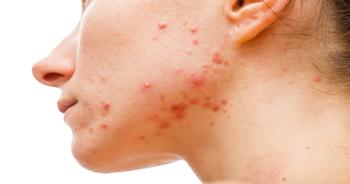
Clascoterone cream 1% is a first-in-class topical acne treatment that targets the androgen receptors in the skin.

Oliceridine (Olinvyk; Trevena, Inc) is an intravenous opioid agonist indicated in adults for management of acute severe pain in a hospital or controlled clinical setting.

Apomorphine hydrochloride (Kynmobi; Sunovion) is a sublingual therapy for the acute intermittent treatment of off episodes in patients with Parkinson disease.
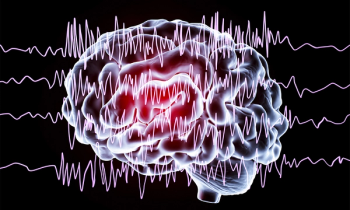
On June 25, 2020, fenfluramine (Fintepla) was approved for the treatment of seizures associated with Dravet syndrome in patients 2 years and older.

The FDA has approved OTC ketotifen fumarate (Alaway Preservative Free; Bausch + Lomb and Eton Pharmaceuticals) ophthalmic solution, 0.035% antihistamine eye drops.

Teva’s 2 digital inhalers for patients with asthma provide insight into objective inhaler use data.

Fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta; GlaxoSmithKline) is indicated for the treatment of asthma in patients aged 18 years and older.

Tramadol hydrochloride (Qdolo; Athena Bioscience) oral solution is indicated for use when pain is severe enough to require an opioid analgesic and for which alternative treatments are inadequate.

Oral azacitidine (Onureg; Celgene) is approved for patients in first remission with acute myeloid leukeimia.

Here's the coronavirus-related news from this week that you should know.

Abbott’s BinaxNOW COVID-19 Ag Card rapid test produces results in 15 minutes.

Amisuplride injection (Barhemsys; Acacia) is the first and only antiemetic to be approved for the rescue treatment of PONV in patients who have failed prior prophylaxis with an agent of a different class.
Mylan has launched the first FDA-approved generic version of Biogen’s dimethyl fumarate (Tecfidera) for relapsing forms of multiple sclerosis.

Risdiplam (Evrysdi; Genentech) is the only SMA therapy that can be administered at home.

Episode 8 of Over the Counter discusses the new and exciting modernizations in the medication decision support space.

Esketamine (Spravato; Janssen) CIII nasal spray is the first FDA-approved antidepressant medication that improves depressive symptoms with the first dose in adults with major depressive disorder.
On May 8, 2020, the FDA granted accelerated approval to selpercatinib (Retevmo; Eli Lilly and Company).

The therapy will also be available for individuals with asthma and suspected COVID-19 infection.

The new app feature will also soon function with smart labels, which can be scanned and read aloud.
Xeris Pharmaceuticals’ new glucagon injection (Gvoke HypoPen), a ready-to-use glucagon formulation in a premixed autoinjector, is now available by prescription.
The FDA has granted emergency use authorization to an additional combination diagnostic test for influenza and COVID-19.
Fostemsavir (Rukobia, ViiV Healthcare) is indicated for use in heavily treatment-experienced adults with multidrug resistant HIV-1 infection.

The fixed-dose combination of pertuzumab and trastuzumab with hyaluronidase (Phesgo; Genentech) can be administered subcutaneously for patients with HER2-positive breast cancer.

A new computer algorithm might be the next step toward accurate prediction of adverse drug reactions.

Officials with the FDA approved fenfluramine (Fintepla, Zogenix) oral solution, CIV for the treatment of seizures associated with Dravet syndrome in patients 2 years of age and older.

The first and only one of its kind, metoclopramide nasal spray offers relief to often debilitating symptoms of the gastrointestinal disease.