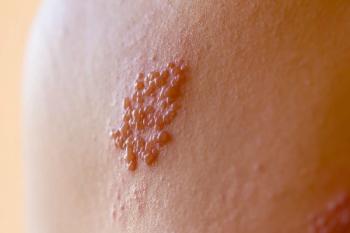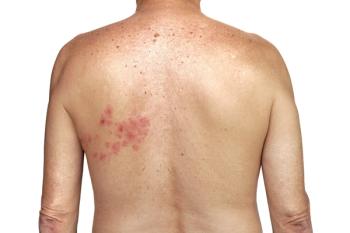
Recurrent Shingles: More Common Than Once Thought
Learn who’s at risk and how vaccination can help.
Once considered a rare occurrence,
The first study,1 based in Minnesota, involved more than 1600 adults 22 years and older with medically documented episodes of HZ. Of these patients, 95 had 105 recurrences of the infection, with a recurrence rate at 8 years of 6.2%.
Researchers found that recurrences were significantly more likely in persons with zoster-associated
“Rates of HZ recurrence appear to be comparable to rates of first HZ occurrence in immunocompetent individuals, suggesting that recurrence is sufficiently common to warrant investigation of vaccine prevention in this group,” the researchers concluded.1 “Zoster vaccine is recommended for prevention of incident cases of HZ. Our high HZ recurrence rates suggest that zoster vaccination should be offered in people who have had an HZ episode to prevent potential recurrences.”
The second study,2 conducted in Korea, concluded that HZ recurrence is much more common than generally expected, and that associated risk factors—including age and gender—can play an important role in predicting recurrence. The recurrence rate in patients over 50 years was 5.8%, compared with 4.5% in younger participants. Women had more frequent recurrence than men.
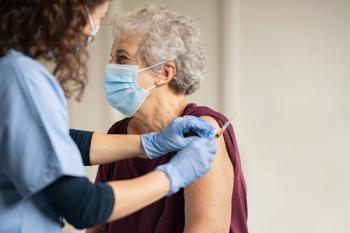
Vaccination is the first line of defense against shingles and its recurrence. In 2017, Shingrix was approved by the
Virtually every person over the age of 50 in the United States carries the
References
- Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P.
Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88-93. Doi:10.4065/mcp.2010.0618 - Kim YJ, Lee CN, Lee MS, et al.
Recurrence rate of herpes zoster and its risk factors: A population-based cohort study. J Korean Med Sci. 2018;34(2):e1. Doi:10.3346/jkms.2019.34.e1
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.