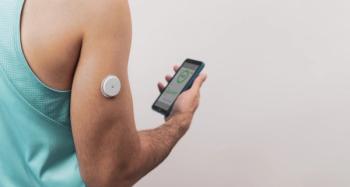
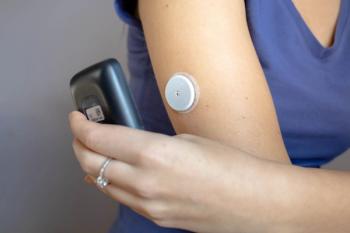
CGM Technology Bridges the Gap in Type 2 Diabetes Care for Underserved Patients
Increased health care access to devices like the continuous glucose monitor (CGM) could improve health outcomes for underserved patients who may otherwise be hindered from their use.
Use of a continuous glucose monitor (CGM) among medically underserved patients can lead to a significant improvement in glycemic control in those being treated for type 2 diabetes (T2D), regardless of treatment therapies used, according to a new study published in Clinical Therapeutics.1
Although the American Diabetes Association recommends either a blood glucose monitor or a CGM for patients with T2D, it also acknowledges the high costs of these devices might render them unaffordable to both insured and uninsured patients. In conducting the current study, investigators aimed to evaluate the effect of initiating continuous glucose monitoring in underserved patients being treated for T2D who otherwise may not have qualified for the devices or could not afford them.
The institutional review board–approved retrospective study, beginning in April 2021, included 41 patients who were provided with a CGM reader and sensors at no cost to them during their standard-of-care clinical visit with their primary care practitioner or a clinical pharmacist. Patients were divided into subgroups based on insulin use, clinical pharmacist and physician management, and physician-only management.
Each patient satisfied criteria of having a diagnosis of diabetes, an interest in using the CGM, and willingness to have the sensor placed during the visit. No restrictions were placed on medication, insurance status, or baseline hemoglobin A1c (HbA1c) level. To emulate a realistic scenario of self-monitored care, patients were provided with replacement sensors and devices and there was no set duration for use of the device.
Primary and secondary outcomes were measured 18 months after the initial CGM was provided to patients, throughout which they were scheduled for regular follow-up appointments with a health care practitioner. The primary outcome is the change in the first HbA1c level after CGM placement, with secondary outcomes including changes in second HbA1c level, use of clinical pharmacist intervention, and changes in HbA1c levels in both insulin- and non–insulin-treated patients.
Significant reductions in the mean first HbA1c level after CGM placement were observed in both insulin-treated (–1.8% [2.8], n = 30) and non–insulin-treated patients (–2% [2.8%], n = 11). The largest reduction in the first HbA1c level after placement was observed in patients who received collaborative care with a clinical pharmacist and the use of a CGM device, measuring at a mean (SD) decrease of –2.5% (2.7%) (n = 26). For patients in a noncollaborative care model, a mean (SD) reduction of –0.8% (1.6%) (n = 15) was observed.
The reduction in HbA1c values was greatest in the first measurement after CGM placement but began to decrease in subsequent measurements. However, findings that patients maintained an overall reduction in HbA1c levels compared with baseline after the third measurement point to the potential of continuous glucose monitoring to have a long-term impact on glycemic control past discontinuation.
Results from the current study, specifically that that observed the greatest improvement in HbA1c levels for patients in a collaborative care model, support and advance past research that found the inclusion of clinical pharmacists in diabetes care led to better glycemic outcomes.2,3 Additionally, this finding suggests that involving a multidisciplinary team in diabetes management may further improve health outcomes.
Investigators noted that, based off evidence that CGM technology improved health outcomes for medically underserved patients, increasing health care access to devices like the CGM can bridge health disparities and protect patients against complications associated with uncontrolled diabetes. Future studies, they continued, should focus on solving financial and logistical challenges related to accessing both CGM and clinical pharmacist team-based care to improve well-being for the millions of Americans diagnosed with T2D.
“These patients would likely not be able to obtain a CGM device under normal care; however, given the improvements in long-term glycemic control that were noted in this study, one could argue that expanding access of these newer technologies may provide a resource to further empower patients in understanding their disease process more effectively and thus influencing their long-term outcomes and glycemic control,” study authors said.
References
1. Powell J, Mulani SR. Partnering for better health: Using continuous glucose monitoring and clinical pharmacist collaboration to improve glycemic control in underserved patients with type 2 diabetes. Clin Ther. Published online October 28, 2023. doi:10.1016/j.clinthera.2023.10.005
2. Deters MA, Laven A, Castejon A, et al. Effective interventions for diabetes patients by community pharmacists: A meta-analysis of pharmaceutical care components. Ann Pharmacother. 2018;52(2):198-211. doi:10.1177/1060028017733272
3. Coutureau C, Slimano F, Mongaret C, Kanagaratnam L. Impact of pharmacists-led interventions in primary care for adults with type 2 diabetes on HbA1c levels: A systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(6):3156. doi:10.3390/ijerph19063156
Newsletter
Pharmacy practice is always changing. Stay ahead of the curve with the Drug Topics newsletter and get the latest drug information, industry trends, and patient care tips.