
The maker of Androgel will face a series of trials after its most recent court loss.

Sometimes customers are people too.

Renflexis has wholesale cost 35% below Remicade.

A new study shows that a pharmacist-physician team can lower inappropriate drug use.




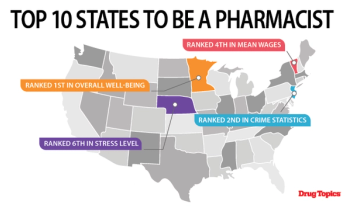
The definitive ranking of the best places to practice pharmacy in America.

The chain is accused of not reporting opioid thefts by its employees.

A new study shows that only a third of children suffering allergic reactions receive epinephrine immediately.

Elevate Provider Network estimates DIR fees in advance

There are elements to consider if you want to sell your pharmacy, or buy one.

Two recent generic cholesterol-lowering drugs are helping reduce health-care costs.

FDA will shorten the review periods for priority generic drugs by two months.

Health-care plans in the Senate include repealing the Prevention and Public Health Fund.

Thousands of faculty and students gathered to hear about the latest in healthcare.

Study finds that adding an antipsychotic to antidepressant therapy may be best for treating depression.

The FDA has already approved 24 new drugs in 2017. Here's what you need to know about the latest 5.

When pharmacists know a patients’ vaccination histories, vaccination rates go up.

The drugs might change, but the people never do. Part 2 of our best of the 70s series.

Mallinckrodt to pay settlement for not reporting orders or keeping accurate records.

An investigation into Actemra (tocilizumab) has uncovered hundreds of serious adverse drug events, but the drug's labeling has not been changed.

Preventing drug-drug interactions with HIV ART

Endari is only the second FDA-approved sickle cell disease drug.

The FDA approved Kevzara for RA patients.

Big-box merchandisers, including supermarkets, employ thousands of pharmacists and offer higher-than-average salaries.

The needs that small pharmacies have-and how they are being met

A pharmacist looks back at what he was told in pharmacy school.